ALL questions listed must be answered to earn credit for completing the text homework. Yes, even the Additional Exercises if they are present for this chapter.
Covalent Compounds
Covalent bond lewis structure calculator November 4, 2020 by Odd-electron molecules have an odd number of valence electrons, and therefore have an unpaired electron. Thus by increasing the potential energy. However, some atoms will not give up or gain electrons easily. For example, consider the Lewis dot structure for carbon dioxide. This is a linear molecule, containing two polar carbon-oxygen double bonds. However, since the polar bonds are pointing exactly 180° away from each other, the bond polarities cancel out, and the molecule is nonpolar. A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. Electrons exist outside of an atom‘s nucleus and are found in principal energy levels that contain only up to a specific number of electrons. Covalent bond lewis structure calculator. November 4, 2020; Posted in Uncategorized 0 Comments; each oxygen atom gets Neon's configuration. This is a qualitative explanation for covalent bond only.
(1) How do atoms form covalent bonds? What is the easiest way to distinguish a covalent bond from an ionic bond?
(2) For each of the following elements, draw their Lewis dot structures.
- Sulfur
- Boron
- Iodine
- Silicon
- Phosphorus

‹ Lewis Dot Structures up Resonance Structures › These tutorials are sponsored by PhySy, the maker of PhySyCalc on iPhone, iPad, or Mac OS, and RMN on Mac OS. PhySyCalc is the only calculator app that let's you use units directly in calculations.
(3) Study the given Lewis structure and provide the following information:
- Draw in any lone pairs that are needed.
- How many bonding electrons does the carbon have? How many does Oxygen have?
- Is the octet of each atom in the molecule satisfied?
- How many single bonds are used in this molecule? How many double bonds?
- How many bonds are shared between the Carbon and Oxygen atoms? How many are shared between the Carbon and each Hydrogen?
(4) Answer each question regarding the following structure:
- Fill in all missing lone pairs
- How many bonding electrons does the Nitrogen have? How many non-bonding electrons does it have?
- How many bonding electrons do each Chlorine have? How many non-bonding electrons do they each have?
- How many electrons, in total, does Nitrogen share with all three Chlorines? Does each atom have an octet?
- Are the bonds formed in this molecule ionic or covalent?
(5) Draw the Lewis dot structures for each of the 7 diatomic elements in their diatomic states. Are these diatomic elements, when one atom is covalently bonded to another of the same element, considered molecules or compounds? Explain using the definition of whichever answer you choose.
(6) For each family or group number, describe the covalent bonding pattern found in organic compounds.
- Halogens
- Group 4A
- Group 6A
- Group 5A
- Noble Gases
(7) Sketch the Lewis configurations for the following molecules. Include all lone pairs in the sketch and determine how many total electrons are in each molecule.
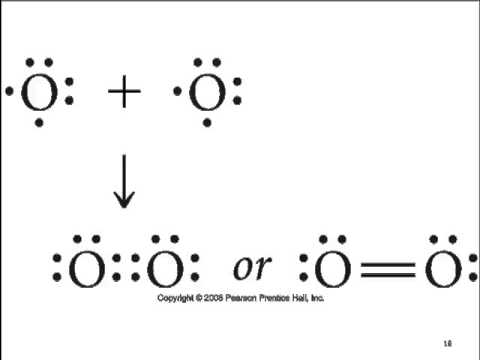
- CH2Cl2
- HCN
- CH2O
- CH3CH2CH3
- CH3COOH (Hint: Both oxygens bond to the second carbon)
(8) What is an expanded octet? Which elements cannot form an expanded octet? Where on the periodic table are the elements that can form expanded octets located?
(9) Give the names of the following covalent compounds.
- N2O4
- SCl6
- CBr4
- P2O5
- ClF7
Three-Dimensional Shapes of Molecules
(10) Include the Lewis dot structure for each given molecule. Give both the electron geometry and molecular shape of the central atom(s).
- CH3F
- H2O
- NH2I
- CH2S
(11) If a central atom has three bonding groups surrounding it, how can you differentiate between the possible molecular shapes it can take on? What electron geometry, molecular shape and bond angles would such a molecule have?
(12) For each of the following molecules, tell whether they are two-dimensional or three-dimensional based on their electron geometry.
- NH3
- H2S
- CH2Cl2
- CH2S
- CO2
(13) What are the approximate bond angles in the given molecules?
Covalent Lewis Dot Structure Calculator Formula
- PBr3
- HNO
- CH3F
- CH2O
- CS2
(14) In each of the following molecules, give the central atom’s molecular shape and the approximate bond angles.
a. O=C=O
b.
c. NH3
d.
e.
Lewis Dot Structure Calc
(15) Use your knowledge of what you’ve learned so far to provide the Lewis dot structures, electron geometry, molecular shape, bond angles, number of lone pair electrons, and the number of shared pair electrons for each molecule given.
- CI2Br2
- NOF
- ClCN
- CH2O
(16) For each of the three carbons in this molecule, provide the requested information.
- The molecular shape of each carbon
- Is this molecule more likely to be two-dimensional or three dimensional?
- What is the bond angle of the CH2?
- What is the H-C-C bond angle?
- What is the C-C-N bond angle?
Modeling Exercises
(17) Construction of acetic acid, CH3COOH
- Obtain two carbons, four hydrogens, and two oxygens with six single-bond sticks and two double-bond sticks
- Sketch the Lewis dot structure to get an idea of how to put it together
- Construct the model to get a three-dimensional visual of acetic acid
- For each carbon atom, how many different electron groups, both bonding and non-bonding, surround them?
- Of those groups and for each carbon, respectively, how many are bonding and how many are non-bonding?
- What is the electron geometry around each carbon? Molecular shape?
- Is the majority of the molecule two-dimensional, or three-dimensional? Justify. (Hint: Try rotating some of the central atoms and looking at it from different angles.)
- What are the bond angles on each central atom (remember to include the oxygen in the -OH as one of the central atoms.)
- Why does one of the two oxygen atoms get treated as a central atom and not the other?
(18) Construction of acetonitrile, CH3CN
- Obtain two carbons, three hydrogens and a nitrogen, four single-bond sticks and three double-bond sticks.
- Sketch the Lewis dot structure of the molecule to determine its structure.
- Construct the model of the molecule for a visual representation.
- How many non-bonding electrons does each carbon have? Bonding electrons?
- Determine the electron geometry and molecular shape of each carbon.
- Is the nitrogen a central atom or not? Explain.
- What is the electron geometry of the nitrogen? What is the molecular shape?
Molecular Polarity
Lewis Dot Structure Calculator With Dots
(19) Between the following pairs, which has the higher electronegativity?
- Oxygen and Nitrogen
- Sulfur and Oxygen
- Chlorine and Bromine
- Carbon and Silicon
- Phosphorus and Nitrogen
(20) Describe the trend in electronegativity as you move left and right across the periodic table. Does electronegativity increase to the right or the left? Now do the same for moving up and down; does electronegativity increase as you go up or down?
Chemistry Element To Lewis Dot Structure Solver
(21) Sketch each of the following molecules to help you determine whether they are polar or non-polar. Draw dipole arrows to indicate polarity in polar molecules.
- CCl4
- PH3
- HOCl
- CS2
- HCOOH
(22) Electronegativity is not the sole decider of polarity within a molecule. Keeping this in mind, determine if the following molecules are polar or not. If not, explain why.
- CH2F2
- CO2
- CH3CH3
- H2O
- CH3OCH3
Intermolecular Forces of Attraction
Lewis Dot Structure Practice Worksheet
(23) Are intermolecular forces considered to be bonds? Explain. Is an H-bond an exception?
Lewis Dot Structure Finder
(24) Which of the three intermolecular forces is the most common? List two other names that can be used to describe this interaction. What is its relative strength compared to the others?
(25) Explain why a dipole-dipole attraction is a stronger force than London Dispersion forces. What is the critical difference that makes this so?
(26) What is the dominant intermolecular forces in each of the following molecules?
- CH2CH2
- CH3OH
- HCN
- CH3OCH3
- NH3
(27) Water (H2O) is one of the most perfect H-bonding molecules we know of. Use your knowledge of H-bonding to explain why solid H2O (ice) floats in liquid H2O while in most other substances, the density of the solids is greater than their respective liquids.
(28) Intermolecular forces are significant for allowing molecules to do things that bonding alone will not allow for. Explain how something like DNA utilizes intermolecular forces to take on its helical shape without relying solely on covalent bonding.